Blowflies’ Perception of Different Sugars – Lab report sample 1
Perception of Different Sugars by Blowflies
ABSTRACT
To feed on materials that are healthy for them, flies (order Diptera) use taste receptors on their tarsi to find sugars to ingest. We examined the ability of blowflies to taste monosaccharide and disaccharide sugars as well as saccharin. To do this, we attached flies to the ends of sticks and lowered their feet into solutions with different concentrations of these sugars. We counted a positive response when they lowered their proboscis to feed.
The flies responded to sucrose at a lower concentration than they did of glucose, and they didn’t respond to saccharin at all. Our results show that they taste larger sugar molecules more readily than they do smaller ones. They didn’t feed on saccharin because the saccharin we use is actually the sodium salt of saccharin, and they reject salt solutions. Overall, our results show that flies are able to taste and choose foods that are good for them.
INTRODUCTION
All animals rely on senses of taste and smell to find acceptable food for survival. Chemoreceptors are found in the taste buds on the tongue in humans (Campbell, 2008), for example, for tasting food. Studies of sensory physiology have often used insects as experimental subjects because insects can be manipulated with ease and because their sensory-response system is relatively simple (E. Williams, personal communication).
Flies are able to taste food by walking on it (Dethier, 1963). Hollow hairs around the proboscis and tarsi contain receptor neurons that can distinguish among water, salts, and sugars, and flies can distinguish among different sugars (Dethier, 1976). These traits enable them to find necessary nutrition.
In this experiment we tested the ability of the blowfly Sarcophaga bullata to taste different sugars and a sugar substitute, saccharin. Because sucrose is so sweet to people, I expected the flies to taste lower concentrations of sucrose than they would of maltose and glucose, sugars that are less sweet to people. Because saccharin is also sweet tasting to people, I expected the flies to respond positively and feed on it as well.
METHODS
We stuck flies to popsickle sticks by pushing their wings into a sticky wax we rubbed on the sticks. Then we made a dilution series of glucose, maltose, and sucrose in one-half log molar steps (0.003M, 0.01M, 0.03M, 0.1M, 0.3M, and 1M) from the 1M concentrations of the sugars we were given.
We tested the flies’ sensory perception by giving each fly the chance to feed from each sugar, starting with the lowest concentration and working up. We rinsed the flies between tests by swishing their feet in distilled water.
We counted a positive response whenever a fly lowered its proboscis. To ensure that positive responses were to sugars and not to water, we let them drink distilled water before each test. See the lab handout Taste Reception in Flies (Biology Department, 2000) for details.
RESULTS
Flies responded to high concentrations (1M) of sugar by lowering their probosces and feeding. The threshold concentration required to elicit a positive response from at least 50% of the flies was lowest for sucrose, while the threshold concentration was highest for glucose (Fig. 1). Hardly any flies responded to saccharin.
Based on the results from all the lab groups together, there was a major difference in the response of flies to the sugars and to saccharin (Table 1). When all the sugars were considered together, this difference was significant (t = 10.46, df = 8, p < .05). Also, the response of two flies to saccharin was not statistically different from zero (t = 1.12, df = 8, n.s.).
DISCUSSION
The results supported my first hypothesis that sucrose would be the most easily detectable sugar by the flies. Flies show a selectivity of response to sugars based on molecular size and structure. Glucose, the smallest of the three sugars, is a monosaccharide.
The threshold value of glucose was the highest in this experiment because a higher concentration of this small sugar was needed to elicit a positive response. Maltose and sucrose are both disaccharides but not with the same molecular weight or composition.
It has been shown that flies respond better to alpha-glucosidase derivatives than to beta-glucosidase derivatives (Dethier 1975). Because sucrose is an alpha-glucosidase derivative, it makes sense that the threshold value for sucrose occurs at a lower concentration than that for maltose. This might also be the reason why sucrose tastes so sweet to people.
My other hypothesis was not supported, however, because the flies did not respond positively to saccharin. The sweetener people use is actually the sodium salt of saccharic acid (Budavari, 1989). Even though it tastes 300 to 500 times as sweet as sucrose to people (Budavari, 1989), flies taste the sodium and so reject saccharin as a salt. T
wo flies did respond positively to saccharin, but the response of only two flies is not significant, and the lab group that got the positive responses to saccharin may not have rinsed the flies off properly before the test.
Flies taste food with specific cells on their tarsal hairs. Each hair has, in addition to a mechanoreceptor, five distinct cells – alcohol, oil, water, salt, and sugar – that determine its acceptance or rejection of the food (Dethier, 1975). The membranes located on the tarsi are the actual functional receptors since it is their depolarization that propagates the stimulus to the fly (Dethier, 1975).
Of the five cells, stimulation of the water and sugar cells induce feeding, while stimulation of the salt, alcohol, and oil receptors inhibit feeding. More specifically, a fly will reject food if the substrate fails to stimulate the sugar or water receptors, stimulates a salt receptor, or causes a different message from normal (e.g., salt and sugar receptors stimulated concurrently) (Dethier 1963).
Flies accept sugars and reject salts as well as unpalatable compounds like alkaloids (Dethier & Bowdan, 1989). This selectivity is a valuable asset to a fly because it helps the fly recognize potentially toxic substances as well as valuable nutrients (H. Cramer, personal communication).
Substances such as alcohols and salts could dehydrate the fly and have other harmful effects on its homeostasis (Dethier, 1976). Thus, flies are well adapted to finding food for their own survival.
ACKNOWLEDGMENTS
I thank Prof. Cramer for help with the t-test and my lab partners for helping me conduct and understand this experiment.
LITERATURE CITED
Campbell, N.A., & J.B. Reece. 2008. Biology, 8th ed. Pearson Benjamin Cummings, San Francisco
Budavari, S., et al. 1989. The Merck Index. Merck & Co., Rahway, NJ.
Biology Department. 2000. Taste Reception in Flies. Biology 101 Laboratory Manual, Hamilton College, Clinton, NY.
Dethier, V.G. 1963. The Physiology of Insect Senses. Methuen & Co., London.
Dethier, V.G. 1976. The Hungry Fly. Harvard University Press, Cambridge.
Dethier, V.G., & E. Bowdan. 1989. The effect of alkaloids on sugar receptors and the feeding behaviour of the blowfly. Physiological Entomology 14:127-136.
Table 1. The average number of flies in each lab group that fed from 0.3M concentrations of each chemical tested. The mean + standard deviation is shown.
chemical tested number of 10 flies responding.
| glucose |
3.2 + 1.5 |
|
|
|
|
| maltose |
7.8 + 2.3 |
|
|
|
|
| sucrose |
8.6 + 2.1 |
|
|
|
|
| saccharin |
0.2 + 0.5 |
|
|
|
|
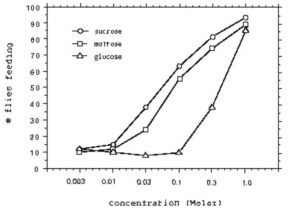
Fig. 1. Taste response curves of flies to different concentrations of the sugars glucose, maltose, and sucrose.

Fig. 2. Chemical formulas of sucrose and maltose (Biology Department, 2000). Glucose is a monosaccharide and is shown as part of each of these molecules.
Lab report sample 2
The Optimal Foraging Theory:
Food Selection in Beavers Based on Tree Species, Size, and Distance
Laboratory 1, Ecology 201
Abstract.
The theory of optimal foraging and its relation to central foraging was examined by using the beaver as a model. Beaver food choice was examined by noting the species of woody vegetation, status (chewed vs. not-chewed), distance from the water, and circumference of trees near a beaver pond in North Carolina.
Beavers avoided certain species of trees and preferred trees that were close to the water. No preference for tree circumference was noted. These data suggest that beaver food choice concurs with the optimal foraging theory.
Introduction
In this lab, we explore the theory of optimal foraging and the theory of central place foraging using beavers as the model animal. Foraging refers to the mammalian behavior associated with searching for food. The optimal foraging theory assumes that animals feed in a way that maximizes their net rate of energy intake per unit time (Pyke et al. 1977).
An animal may either maximize its daily energy intake (energy maximizer) or minimize the time spent feeding (time minimizer) in order to meet minimum requirements. Herbivores commonly behave as energy maximizers (Belovsky 1986) and accomplish this maximizing behavior by choosing food that is of high quality and has low-search and low-handling time (Pyke et al. 1977).
The central place theory is used to describe animals that collect food and store it in a fixed location in their home range, the central place (Jenkins 1980). The factors associated with the optimal foraging theory also apply to the central place theory.
The central place theory predicts that retrieval costs increase linearly with distance of the resource from the central place (Rockwood and Hubbell 1987). Central place feeders are very selective when choosing food that is far from the central place since they have to spend time and energy hauling it back to the storage site (Schoener 1979).
The main objective of this lab was to determine beaver (Castor canadensis) food selection based on tree species, size, and distance. Since beavers are energy maximizers (Jenkins 1980, Belovsky 1984) and central place feeders (McGinley and Whitam 1985), they make an excellent test animal for the optimal foraging theory. Beavers eat several kinds of herbaceous plants as well as the leaves, twigs, and bark of most species of woody plants that grow near water (Jenkins and Busher 1979).
By examining the trees that are chewed or not-chewed in the beavers’ home range, an accurate assessment of food preferences among tree species may be gained (Jenkins 1975). The purpose of this lab was to learn about the optimal foraging theory. We wanted to know if beavers put the optimal foraging theory into action when selecting food.
We hypothesized that the beavers in this study will choose trees that are small in circumference and closest to the water. Since the energy yield of tree species may vary significantly, we also hypothesized that beavers will show a preference for some species of trees over others regardless of circumference size or distance from the central area.
The optimal foraging theory and central place theory lead us to predict that beavers, like most herbivores, will maximize their net rate of energy intake per unit time.
In order to maximize energy, beavers will choose trees that are closest to their central place (the water) and require the least retrieval cost. Since beavers are trying to maximize energy, we hypothesized that they will tend to select some species of trees over others on the basis of nutritional value.
Methods
This study was conducted at Yates Mill Pond, a research area owned by the North Carolina State University, on October 25th, 1996. Our research area was located along the edge of the pond and was approximately 100 m in length and 28 m in width.
There was no beaver activity observed beyond this width. The circumference, the species, status (chewed or not- chewed), and distance from the water were recorded for each tree in the study area. Due to the large number of trees sampled, the work was evenly divided among four groups of students working in quadrants. Each group contributed to the overall data collected.
We conducted a chi-squared test to analyze the data with respect to beaver selection of certain tree species. We conducted t-tests to determine (1) if avoided trees were significantly farther from the water than selected trees, and (2) if chewed trees were significantly larger or smaller than not chewed trees. Mean tree distance from the water and mean tree circumference were also recorded.
Results
Overall, beavers showed a preference for certain species of trees, and their preference was based on distance from the central place. Measurements taken at the study site show that beavers avoided oaks and musclewood (Fig. 1) and show a significant food preference (x2=447.26, d.f.=9, P<.05).
No avoidance or particular preference was observed for the other tree species. The mean distance of 8.42 m away from the water for not-chewed trees was significantly greater than the mean distance of 6.13 m for chewed trees (t=3.49, d.f.=268, P<.05) (Fig. 2).
The tree species that were avoided were not significantly farther from the water (t=.4277, d.f.=268, P>.05) than selected trees. For the selected tree species, no significant difference in circumference was found between trees that were not chewed (mean=16.03 cm) and chewed (mean=12.80 cm) (t=1.52, d.f.=268, P>.05) (Fig. 3).
Discussion
Although beavers are described as generalized herbivores, the finding in this study related to species selection suggests that beavers are selective in their food choice.
This finding agrees with our hypothesis that beavers are likely to show a preference for certain tree species. Although beaver selection of certain species of trees may be related to the nutritional value, additional information is needed to determine why beavers select some tree species over others.
Other studies suggested that beavers avoid trees that have chemical defenses that make the tree unpalatable to beavers (Muller-Schawarze et al. 1994). These studies also suggested that beavers prefer trees with soft wood, which could possibly explain the observed avoidance of musclewood and oak in our study.
The result that chewed trees were closer to the water accounts for the time and energy spent gathering and hauling. This is in accordance with the optimal foraging theory and agrees with our hypothesis that beavers will choose trees that are close to the water.
As distance from the water increases, a tree’s net energy yield decreases because food that is farther away is more likely to increase search and retrieval time. This finding is similar to BelovskyÃs finding of an inverse relationship between distance from the water and percentage of plants cut.
The lack of any observed difference in mean circumference between chewed and not chewed trees does not agree with our hypothesis that beavers will prefer smaller trees to larger ones. Our hypothesis was based on the idea that branches from smaller trees will require less energy to cut and haul than those from larger trees.
Our finding is in accordance with other studies (Schoener 1979), which have suggested that the value of all trees should decrease with distance from the water but that beavers would benefit from choosing large branches from large trees at all distances. This would explain why there was no significant difference in circumference between chewed and not-chewed trees.
This lab gave us the opportunity to observe how a specific mammal selects foods that maximize energy gains in accordance with the optimal foraging theory. Although beavers adhere to the optimal foraging theory, without additional information on relative nutritional value of tree species and the time and energy costs of cutting certain tree species, no optimal diet predictions may be made.
Other information is also needed about predatory risk and its role in food selection. Also, due to the large number of students taking samples in the field, there may have been errors which may have affected the accuracy and precision of our measurements. In order to corroborate our findings, we suggest that this study be repeated by others.
Conclusion
The purpose of this lab was to learn about the optimal foraging theory by measuring tree selection in beavers. We now know that the optimal foraging theory allows us to predict food-seeking behavior in beavers with respect to distance from their central place and, to a certain extent, to variations in tree species. We also learned that foraging behaviors and food selection is not always straightforward.
For instance, beavers selected large branches at any distance from the water even though cutting large branches may increase energy requirements. There seems to be a fine line between energy intake and energy expenditure in beavers that is not so easily predicted by any given theory.
Literature Cited
Belovsky, G.E. 1984. Summer diet optimization by beaver. The American Midland Naturalist. 111: 209-222.
Belovsky, G.E. 1986. Optimal foraging and community structure: implications for a guild of generalist grassland herbivores. Oecologia. 70: 35-52.
Jenkins, S.H. 1975. Food selection by beavers:› a multidimensional contingency table analysis. Oecologia. 21: 157-173.
Jenkins, S.H. 1980. A size-distance relation in food selection by beavers. Ecology. 61: 740-746.
Jenkins, S.H., and P.E. Busher. 1979. Castor canadensis. Mammalian Species. 120: 1-8.
McGinly, M.A., and T.G. Whitham. 1985. Central place foraging by beavers (Castor Canadensis): a test of foraging predictions and the impact of selective feeding on the growth form of cottonwoods (Populus fremontii). Oecologia. 66: 558-562.
Muller-Schwarze, B.A. Schulte, L. Sun, A. Muller-Schhwarze, and C. Muller-Schwarze. 1994. Red Maple (Acer rubrum) inhibits feeding behavior by beaver (Castor canadensis). Journal of Chemical Ecology. 20: 2021-2033.
Pyke, G.H., H.R. Pulliman, E.L. Charnov. 1977. Optimal foraging. The Quarterly Review of Biology. 52: 137-154.
Rockwood, L.L., and S.P. Hubbell. 1987. Host-plant selection, diet diversity, and optimal foraging in a tropical leaf-cutting ant. Oecologia. 74: 55-61.
Schoener, T.W. 1979. Generality of the size-distance relation in models of optimal feeding. The American Naturalist. 114: 902-912.
*Note: This document was modified from the work of Selena Bauer, Miriam Ferzli, and Vanessa Sorensen, NCSU.
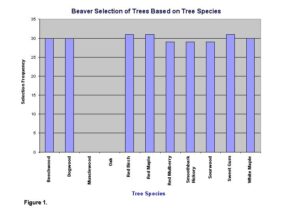
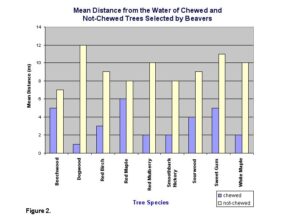
Lab report sample 3
Examination of Protozoan Cultures to Determine Cellular Structure and Motion Pattern
Abstract
Protozoans are unicellular eukaryotes with either plant- or animal-like characteristics. Through careful observation, we analyzed various protozoan cultures in order to identify characteristics associated with cell structure and movement of these one-celled organisms.
We found that Protists exhibit certain characteristics that allow them to be categorized into different groups, mainly determined by their locomotion patterns.
Despite differences in locomotion and the varying plant-like and animal-like organelles, all protists share key characteristics and functions that allow them to feed, grow, and reproduce–processes essential for survival and common to complex organisms.
Introduction
Unicellular eukaryotes belong to the kingdom Protista, and are often referred to as “protists†or “protozoans.†The name “protozoan†means “first animal,†but eukaryotes may display either plant or animal-like characteristics, or a combination of both.
Although unicellular, they have a nucleus and membrane-bound organelles, making them functionally complex despite their small size. Each small protist is a self-supporting unit, carrying out all the processes for survival in just one cell.
They thrive on moisture and can be found on moist soil and in fresh and marine bodies of water. There are about 30,000 known species of protozoans, commonly classified according to their movement patterns as sarcodines—moving with false feet called pseudopodia or, flagellates—moving with whip-like structures known as flagella, ciliates—moving with short hairs known as cilia, and sporozoans—with no movement.
They all have varying shapes, sizes, and survival strategies. For example, some may “hunt†small particles of food such as bacteria or algae; whereas others may be parasitic, inhabiting larger organisms. Despite their differences, all protists have several characteristics in common.
In addition to a nucleus or nuclei to house their genetic material, most protists have mitochondria for metabolic functions, and vacuoles for digestion and excretion. With the help of these and other cellular structures, protists may feed, grow, and reproduce.
In this lab we observed select examples of protists in order to identify their cellular structures, and determine to which group of protista they belong based on their form of movement. We also made drawings of our observations using light and dissection microscopes to practice proper microscopy skills, including making wet-mount slides and cell sizing.
By observing, drawing, and classifying protista, we learned about the cell structure and movement patterns of these one-celled organisms. We also learned about the differences and similarities of various protist cells.
Since we will observe how protists move, it will be interesting to figure out patterns of locomotion. For example, what happens when the protist encounters an obstacle? Does motion change when the organism is feeding? How does motion relate to where the organism lives? What characteristics do the protists exhibit: plant, animal, or both? Do the plant/animal characteristics influence motion patterns?
Methods
Three protists were chosen for observation. See the list of protists below to choose three samples. For each of the protists, a pipette was used to extract a few drops of culture from the culture jar.
The drops of culture were placed on a clean microscope slide and covered with a slide cover slip. Using a light microscope, each protist was examined at different magnifications until the best field of view was found for identifying cellular structures.
The color, shape, and motion cellular structures was noted. Each of the protists was drawn and the drawings were labeled. Field-of-view, magnification, and cell size was noted on the drawings, along with the organism’s name and protist group.
| Protists available for observation: |
Euglena
Paramecium
Difflugia
Blepharisma
Didinium
Amoeba
Stentor
Spirostomum
Vorticella
Volvox
Bursaria |
Results
All protists that were selected had features in common, but they all moved differently. The example protists were: Euglena, Paramecium, and Amoeba. Euglena moved with a flagellum and so is classified as a flagellate (see Fig. 1). Paramecium moved with cilia and so is classified as a ciliate (see Fig. 2). Finally, Amoeba moved with a pseudopod, and so is a sarcodine (see Fig. 3). All three protists had a nucleus, as expected, but the Paramecium had two nuclei, a micronucleus and a macronucleus.
The Paramecium and Amoeba both had food and contractile vacuoles, but these were lacking in the Euglena. All protists had animal-like characteristics in terms of their movements and feeding patterns. Of the three, Euglena was the only one that had chloroplasts, an organelle common in plants.
Discussion
Protists seem to share certain characteristics even when they are classified into different groups. Their organelles are a mixture of animal and plant structures, but they all have nuclei, a feature which distinguishes Protists from other unicellular organisms.
The protists’ motion was consistent with their locomotion organ: cilia, flagella, or pseudopod. This motion was very clear under the light microscope, but interactions of protists with others in the culture jar were better observed using the dissection scope. The Amoeba moves by extending part of its cell. This extruding part is the pseudopod, and allows the Amoeba to drag itself from one place to another (see Fig. 3).
Its movement is slow, and changing directions is just a matter of extending a pseudopod in a new direction. Amoebas do not seem to have a particular shape, with the exception of the pseudopodia that consistently protrude from the cell. This shapeless but ever shifting quality of the Amoeba’s shape allows it to surround, engulf, and ingest its food by a process called phagocytosis.
Paramecia are smaller than Amoebas. They move with the help of microscopic hair-like structures called cilia, which act like oars to push them through the water. They swim by rotating slowly and changing directions often. If the Paramecium comes upon an obstacle, it stops, swims backwards, and then angles itself forward on a slightly different course.
Cilia help the Paramecium move as well as feed. When the Paramecia feed, it does so by drawing its food into a funnel-shaped opening called the oral groove that is lined with cilia (see Fig. 2). The oral groove is like a mouth, taking food in with the help of cilia, which direct and move the food inward.
The Euglena moves rapidly, using its flagellum to propel itself through the water rather quickly, shifting directions with whip-like movements. Unlike the Amoeba and the Paramecium, the Euglena has plant-like characteristics. It is sometimes referred to as a “plant-like†protist.
The organelle that gives it this plant-like quality is the chloroplast (see Fig. 1), a green organelle responsible for carrying out photosynthesis in plants. The Euglena senses light with a light-sensitive organelle called the “eyespot,†which directs the organism to a light source strong enough for photosynthesis to occur. Since it can undergo photosynthesis, Euglena is able to make its own food just like plants.
The three protists examined in this lab are examples of protists that use specialized structures for locomotion. Although the Euglena has some “plant-like†characteristics, all protists mentioned above, exhibit animal-like movements. These protists exemplify the animal-like and motile types of protozoans. As compared to other protists, the animal-like features of the protists we observed allow them to be motile. Their motility comes in handy for moving about their environment and finding food. T
hey may be contrasted to another class of protist, the sporozoans. Sporozoans have no form of locomotion and are primarily parasitic, ingesting their food by absorption through their cell membranes. No matter what type of locomotion a protist uses, all protists must be able to carry out the metabolic functions of multicellular organisms. Based on the observations in this lab, protists are very small yet highly complex.
They have all the organelles necessary for a variety of functions such as digestion, excretion, reproduction, respiration, and movement. Protists are self-supporting “one cell factories†churning out all the processes that are usually carried out by a highly-organized network of cells.
Conclusion
In this lab I learned about the structure and function of the smallest eukaryotic organisms, the unicellular protists. Although very tiny, these organisms are very complex, housing all the necessary life tools in one single cell. This shows that the complexity of an organism is not necessarily related to its size. I also learned to identify and classify different types of protists.
I was able to observe locomotion patterns as well as other characteristic features. In doing so, I gained useful microscopy skills such as making wet mount slides, finding the proper magnification for viewing, and drawing microscope observations with all the proper labels.
Lab report sample 4
The effects of jumpamine chloride on jumping performance in two species of frogs of the genus Rana
Leo Lizardgazer 1997.
Introduction
Jumpamine chloride (JCl) is a natural waste product of muscle metabolism in many species of frogs (Phrogsucker et al. 1957). In addition it was reported by Phrogsucker et al. (1957) that up to 60% of this chemical is reabsorbed from the bladder before excretion.
This result led to a number of studies attempting to identify the advantage of reabsorption of this product. One recent study showed that injection of JCl into the bloodstream increased muscle mass in the leopard frog Rana pipiens (Hylaflex and Smith1988).
Anurheight (1990) was the first to demonstrate an actual improvement in performance capability, by showing that swimming performance in the African clawed frog Xenopus laevis was improved by adding JCl to the diet. Subsequently, in another study, tree frogs (Hyla cinerea) that had been injected with JCl were found to have measurably larger leg muscles and were able to climb higher and more quickly than those that had not (Smith 1992).
The mechanism for the action of JCl on muscle growth and muscle contraction is still unknown. It may interact with enzymes involved in muscle contraction as proposed by Smith (1992) or it may directly act on the mechanical properties of the muscles themselves.
This has been proposed for the action of the hormone gogetemall on muscle growth in the tree lizard Philanthropus fabricus (Herpbrain and Phutz 1992). Concerns over a recent increase in doping with JCl in frog jumping contests suggests that further study of the effect of JCl on jumping performance is necessary (Twainson 1990).
The present study was carried out in order to see if JCl had any direct effects on jumping performance in frogs of the genus Rana. We hypothesized that the increased muscle mass shown in earlier studies (Hylaflex and Smith 1988) would result in improved jumping distance. We predicted, therefore, that frogs injected with JCl should have larger muscles and jump further than frogs that had not been injected with JCl.Â
Such a result would suggest the biological function of JCl reabsorption. We also investigated the influence of temperature in modifying the effects of JCl on jumping performance. Demonstrating temperature effects would shed light on the underlying mechanism involved in the changes in muscle induced by JCl.
Based on earlier studies (Smith 1992) we hypothesized that JCl acts by affecting the enzymes associated with muscle contraction. If this is the case, and since increases in temperature also often lead to increases in enzyme activity, we predicted that jumping distance will improve exponentially with increases in temperature.
The effects of JCl on jumping performance were tested by injecting the drug into the bloodstream of the frogs and measuring average jumping distance under specific conditions. The effects of temperature on jumping distance were evaluated by carrying out the same experiments at a range of different ambient temperatures.
The study was conducted on two different species of frogs, the leopard frog (Rana pipiens) and the Bullfrog (Rana catesbeiana), to see if the effects observed were species-specific or more general in nature.
Materials & Methods
The effect of JCl on jumping distance:
Ten specimens of Rana pipiens were injected with 1.0 ml. of a 10% JCl solution. Ten control frogs were given injections of 1.0 ml of a .9% NaCl solution. All frogs were maintained in 3 m square tanks at 250C for 1 day in 1 inch of water.
At this time each frog was placed on an open floor and induced to jump 3 times by slapping the ground behind the frog. The jumping distance was defined as the average of the 3 jumps. The same procedure was repeated using Rana catesbeiana.
The effect of temperature on jumping distance:
Each of the JCl treated frogs was placed in a 3 m square temperature controlled tank containing 1 inch of water and ranging from 0 to 900C in intervals of 100Â C. One control frog was placed in the tank with each treated frog. The frogs were left in the temperature controlled tanks for 24 hours, and then tested, as above, for jumping performance.
Results
The effects of JCl on jumping distance was studied in two species of frogs of the genus Rana at 250 C and subsequently on frogs that had been maintained over a broad range of temperatures.
Effect of JCl on jumping distance at 250Â C:
As shown in Table 1 the jumping distance for the control Rana pipiens was 2.3 m and for the JCl treated Rana pipiens was 4.2 m.  In Rana catesbeiana the jumping distance for the control frogs was 2.6 m and for the JCl treated frogs was 2.5 m. It is clear from Figure 1 that JCl had a striking impact on Rana pipiens, but had little or no effect on Rana catesbeiana .
The effect of temperature on jumping distance:
As seen in Table 2 the greatest jumping distance of Rana pipiens was 9.0 m at 900 C and the lowest jumping distance was 2.5 m at 00 C. As seen in Table 2 for Rana catesbeiana the greatest jumping distance was 9.1 m at 900 C however the lowest jumping distance was 2.0 m at 300 C. The relationship between temperature and jumping distance is shown for Rana pipiens in Figure 2.
The same relationship for Rana pipiens is shown in Figure 3. It is clear from Figure 2 that for R. pipiens jumping distance increases linearly with temperature. For R. iwanna temperature also affects jump distance in an approximately linear fashion, but does not begin to have an effect until the temperature exceeds 30 0C. At temperatures lower than 30 0C jumping distance varies only slightly between 2.0 m and 2.5 m.
In addition it was noted that the treated frogs exposed to higher temperatures exhibited a measurable weight loss.
Table 1
The effect of JCl on jumping distance in Rana pipiens and Rana catesbeiana at 250 C
| Frog type |
Jumping distance (m) |
| Rana pipiens (JCl treated) |
4.2 |
| Rana pipiens (control) |
2.3 |
| Rana catesbeiana (JCl treated) |
2.5 |
| Rana catesbeiana (control) |
2.6 |
Table 2
The effect of temperature on jumping distance in Rana pipiens
| Temperature (0Â C) |
Jumping distance (m) |
| 0 |
2.6 |
| 10 |
3.1 |
| 20 |
4.0 |
| 30 |
4.9 |
| 40 |
5.1 |
| 50 |
6.0 |
| 60 |
6.8 |
| 70 |
7.5 |
| 80 |
9.0 |
| 90 |
9.2 |
Table 3
The effect of temperature on jumping distance in Rana catesbieana
| Temperature (0Â C) |
Jumping distance (m) |
| 0 |
2.5 |
| 10 |
2.5 |
| 20 |
2.2 |
| 30 |
2.0 |
| 40 |
3.8 |
| 50 |
4.2 |
| 60 |
6.0 |
| 70 |
7.0 |
| 80 |
7.9 |
| 90 |
9.1 |



Discussion
The present study was carried out in order to see if JCl had any direct effects on jumping performance in frogs of the genus Rana. We hypothesized that the increased muscle mass shown in frogs with higher levels of JCl would result in improved jumping distance. We predicted, therefore, that frogs injected with JCl should have larger muscles and jump further than frogs that had not been injected with JCl. JCl has the clear effect of increasing jump distance in both frog species (see Fig. 1, 2 and 3). These results support our original hypothesis that JCl would improve jumping performance.
The influence of temperature in modifying the effects of JCl on jumping performance was also evaluated. It was hypothesized that JCl acts by affecting the enzymes associated with muscle contraction. If this is the case it was predicted that jumping distance would increase exponentially with increases in temperature. Jumping distance is clearly temperature dependent.
However, there are differences between the species in how this effect appears. For example, in R. catesbeiana no increase in performance occurs until the temperature exceeds 300C. This explains why no difference in jumping distance was observed at room temperature (Fig. 1).
The nature of the relationship between temperature increase and JCl effects on jumping distance was not consistent with our original hypothesis regarding the molecular mechanism of action of JCl. We had proposed that JCl affects the activity of certain enzymes. This led us to predict an exponential increase in jumping distance with temperature.
The linear increase we observed is not consistent with the proposed mechanism. It suggests that JCl may be directly acting on the mechanical properties of the muscles themselves. Such a mechanism has been proposed for the action of the hormone gogetemall in the tree lizard Philanthropus fabricus (Herpbrain and Phutz, 1992). This suggests an interesting line of study for future experiments in which JCl- would be administered to isolated muscle preparations and its direct effects on contractile elements observed directly.
The observation that weight loss occurs when treated frogs are exposed to higher temperatures also suggests an effect of JCl on the overall metabolism of frogs. We are currently carrying out a study to test the direct effects of JCl on metabolic rate.
The results described above are important for understanding the role of JCl in the natural biology of these frogs. In Rana pipiens, reabsorption of JCl will clearly lead to increased jumping ability which can be expected to improve its survival chances. Moreover this advantage will occur at temperatures during which it is normally active (20-40 0C). The comparison with R. catesbeiana is interesting however. R. catesbeiana is not normally active above 300C.
Nonetheless, R. catesbeiana absorbs JCl from its bladder. This strongly suggests that improved jumping performance alone cannot account for the evolution of the general tendency of frogs to reabsorb this substance. It would be very interesting to compare the relative amount of reabsorption in frogs active at temperatures where the effects on jumping performance occur versus those where it does not.
The results presented here also have serious implications for the use of JCl in frog jumping contests. Twain son (1990) expressed concern that the increased occurence of doping with this drug in frog jumping contests may have dire consequences for the sport. Here we clearly show that this drug has the potential to influence the outcomes.
The seriousness of the effect on the results clearly depends on the temperature at which contests are held, as well as the species involved. Moreover we have recently found that use of JCl compromises the health of our frogs. Our results support the conclusions of Twainson (1990), and suggest that government regulation and drug testing may be in order.
References
Anurheigh L. 1990. The swimming abilities of frogs. New York: Academic Press.
Herpbrain KZ, Phutz IMA. 1992. Why do hot lizards run faster? Journal of Hyperactive Reptiles 26: 10-23
Hylaflex JD, Smith AP. 1988. Is JCl a frog muscle builder? Journal of Frog Kinesiology 188 (23):1-29
Phrogsucker RQ, Krabby SD, Kidding RU. 1957. Reabsorption of certain biochemicals from the frog bladder: Peeing is believing. Copious 12(3): 134-152
Smith AP. 1992. JCl and climbing ability in tree frogs. Biotropica 23:1-4
Twainson CR. 1990. Frog jumping and drugs: an institution under attack. The American Unnaturalist 12:1-6
